Outline of soap production
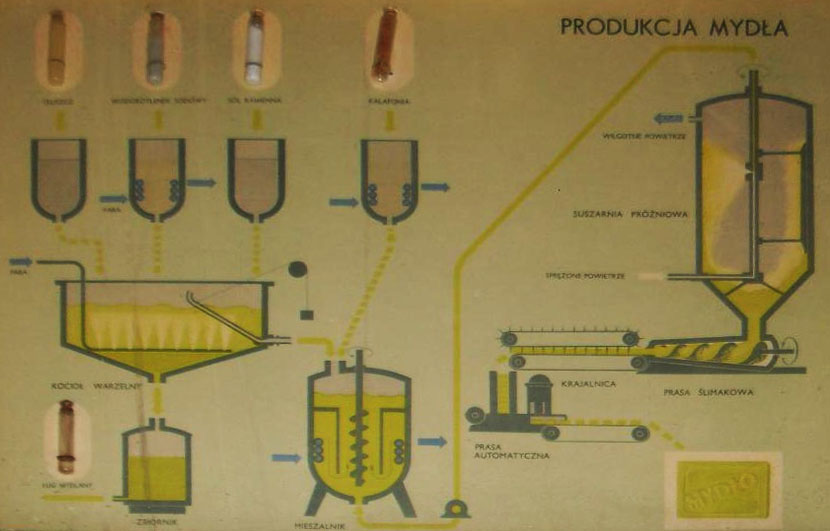
The soap production process begins with the preparation of raw materials. The composition of fats and other ingredients intended for saponification is the so-called. the warp.
The actual soap making process can be divided into two stages:
• brewing or boiling soap, the purpose of which is to saponify the fatty raw materials and create a soap mass;
• confectioning, which gives the soap shape and properties, required by the consumer.
In the first stage, fat with a small amount of caustic soda (aqueous concentrated NaOH solution) it is heated with water until the mass sticks together. The remaining fat and more lye are then gradually added, constantly stirring. In periodic devices, this process takes approx. 2-5 hours and is called saponification.
Then the soap process is carried out, consisting in adding water with constant stirring until the moment, until the soap mass begins to pull like a thread.
In order to separate the soap from the rest of the ingredients, the soap mass is salted out. It consists in gradually adding brine to the soap mass (concentrated salt solution) or solid salt, constantly mixing the contents. Soap precipitates out of solution, which flows upwards. After some time, it is delaminated into a soapy layer and the lye of the trousers, containing glycerin. The lye of the trousers is removed, and the action of salting out is repeated many times. The obtained product is the so-called. send.
The saponification of the acid raw materials is carried out in parallel in a second device, included in the soap, such as rosin or fatty acids. After salting out, auxiliary salt is obtained, which mixes with the base rate.
The mixture of primary and secondary salt is ground. It consists in slowly adding water, with vigorous stirring of the contents with steam, and adding a little sodium hydroxide solution. The salting-out and grinding operation is repeated several times to remove glycerin and other impurities from the soap., especially colorful.
After the last, the final stratification results in salt and the so-called. glue. Wysół descends, cools and dries. Depending on the applied cooling and drying processes as well as mechanical processing, the soap can be obtained directly in pieces or in the form of chips, which can be rubbed and squashed into soap bars or formed into cubes. The soap obtained in this way is called core soap, which may be a finished product or subject to confectioning.
The most important parameters, which determine the quality of the core soap and should be controlled during the production process are:
– free caustic alkali content,
– unsaponified fat content,
– chloride content.
The free caustic alkali content is the residue of unreacted sodium hydroxide in the soap. Soda and potassium lye are caustic substances, cause skin irritation, they are especially dangerous to the eyes, because in contact with them they can lead to permanent loss of vision. Therefore, this parameter must be strictly controlled, especially in the production of soaps for children.
The unsaponifiable fat content means the residual fat remaining in the soap. Unsaponified fats reduce the detergency of the soap, they change its smell unfavorably, especially for unsaturated fats, which go rancid.
The chloride content is due to the residual table salt (NaCl) in soap after salting out processes. Excessive amount of them increases the brittleness of the soap.