Washing mechanism
The purpose of washing is to remove dirt from the surface of the washed material. Washing takes place via a washing bath, which is an aqueous solution of soap or detergent.
Dirt is a very complex substance. Airborne pollutants, soil, waters, containing soot, soil particles, fats, Mineral oils and other substances are deposited on the human body, his clothes and objects of use. In contact with sweat, secreted through the skin, are bonded and tightly glued to the surface of the fabrics. Some of the fabrics become soiled with nutrients, containing fatty and protein substances difficult to remove.
Individual types of dirt are removed with the use of appropriate surfactants. Unsuitable detergent is used, due to the type of dirt and the type of washed material, does not guarantee the desired effect, and may even damage the washed material.
Washing is a complex physicochemical process. Mechanisms, which contribute to the removal of dirt are:
• reduction of the surface tension of the washing bath;
• creating coats on the surface of dirt, binding the dirt with the washing bath;
• generation of electric charges on the surface of the washed product and on the surface of dirt.
One can find out about the existence of surface tension, by putting a steel dry needle on the water surface. If done carefully, the needle does not sink and is held by an invisible film on the surface of the water. The surface tension value is different for different liquids, np. gasoline has a surface tension twice as low as water. The surface tension decreases with increasing temperature and changes as various substances are dissolved in the liquid, np. an admixture of ether or alcohol reduces the surface tension of water, while dissolving sugar or salt increases them.
Substances that change the surface tension of a liquid are called surfactants. These include, among others. soaps and detergents, which significantly lower the surface tension of water.
All chemical compounds show surface activity, whose molecules are amphiphilic, i.e.. they consist of a hydrophilic and a hydrophobic part (drawing).
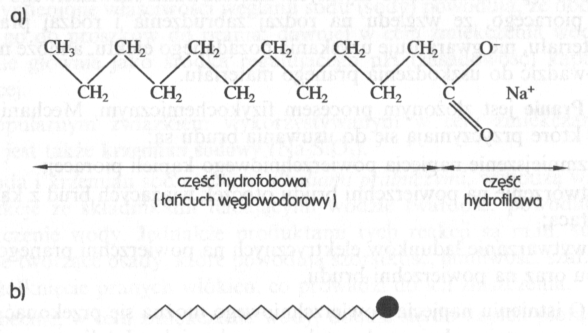
Drawing. Amphiphilic soap molecule: a) chemical formula, b) simplified drawing
The hydrophilic part, constituting the so-called. a hydrophilic group, has this property, that it is attracted to water molecules, which may approach and surround the hydrophilic part. Hydrophobic part, being a hydrophobic group, it is repelled by water molecules, thus the water molecules remain separated from this group. The hydrophobic group additionally has this property, that since it does not mix with water, can combine with substances, which do not dissolve in water, np. with gasoline, with oils, fats. The abovementioned properties of amphiphilic molecules cause their specific behavior in aqueous solutions.
Molecules of a surfactant, in the washing bath change their position. If they are on the border of two phases, np. on the surface of the solution, then the hydrophobic group is pushed outward, as a result of repulsion by water molecules, while the hydrophilic group, attracted by water, it is directed deep into the solution and as if it is anchored in the solution (drawing).
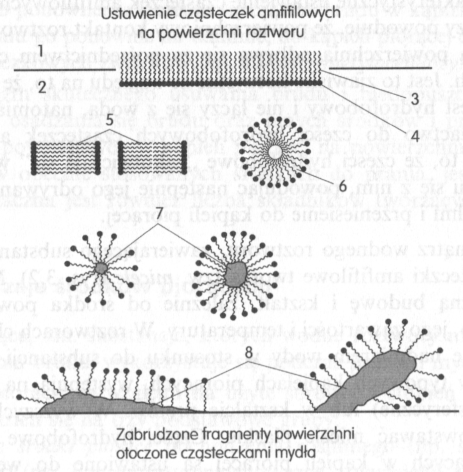
Drawing. Structural elements of the washing bath, decisive for the course of the dirt removal process: 1 - air, 2 - solution, 3 - hydrophobic parts of amphiphilic molecules, hydrocarbon chains, 4 - hydrophilic parts of amphilic particles, 5 - flat micelles, 6 - spherical micelles, 7 - dirt particles suspended in the solution, 8 - bride.