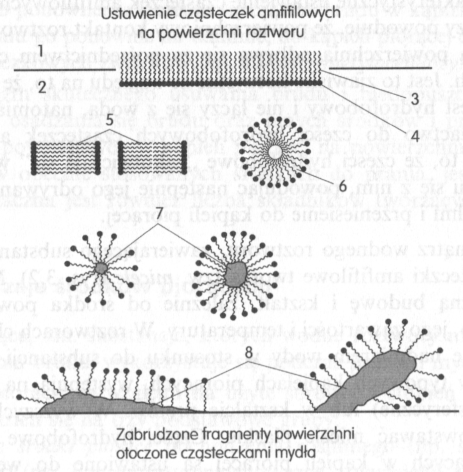
As a result, the surfactant molecules are arranged perpendicularly on the surface of the solution, that is, at the border of the liquid and gas phases. Arranged in this way, the particles closely cover the surface of the solution. If the number of these molecules is large, the liquid tends to increase its surface area, thus it has an effect opposite to surface tension, significantly reducing them. Thanks to this, each additional surface, which will come into contact with the wash liquor will be coated with particles of the surfactant.
The washing bath has the ability to penetrate into various "inaccessible" places of the washed material. It can penetrate the gaps between the dirt and the fiber of the washed material, causing the soil to detach from the fabric and transfer it to the wash liquor. The mechanical rubbing of the fabric surface greatly facilitates and speeds up this operation.
The characteristic arrangement of amphiphilic molecules on the surface of the liquid causes, that the surface contact of the washing solution with the dirty surface is via the hydrophobic parts. This is a favorable phenomenon due to this, that dirt is hydrophobic by nature and does not mix with water, on the other hand, it has an affinity for parts of the hydrophobic amphiphilic molecules. Means, that the hydrophobic parts "anchor" in the dirt upon contact with it, then causing it to detach from the surface to be washed and transfer to the wash bath.
Inside the aqueous solution, containing an amphiphilic substance, amphiphilic molecules form the so-called. please. Micelles can have a different structure and shape, depending on the surfactant, its content and temperature. In solutions characterized by an excess of water in relation to the amphiphilic substance, so in typical washing baths, spherical micelles are generally present (spherical) or in the shape of bars. Flat micelles may form at higher concentrations. The hydrophobic parts in the micelles present in the wash liquor face inwards. Outside, the surface of the micelles is hydrophilic and combines with water molecules. Dirt particles combined with hydrophobic groups, going to the washing bath, they are dispersed in it or closed in micelles, so they can remain in solution and be removed with it.
The effectiveness of washing is also determined by the effects of the electrostatic nature inside the washing bath. Fabric spots, from which the dirt particles have been torn off are immediately covered by the detergent particles. If the detergent dissociates into ions, then an equal charge is created on the surface of the fibers and on the surface of the dirt, which helps to separate them from each other and prevents the repeated deposition of dirt on the fabric. This process can be assisted by introducing multivalent ions into the wash liquor, e.g.. alkali polyphosphates or polysilicates.
Covering the fiber with particles of a surfactant depends on the chemical nature of the fiber and has an influence on the repeated deposition of dirt on the fabric. To make the surface of the fibers and dirt with a layer of surfactant effective and to prevent the dirt particles from reattaching in the washing bath and depositing it again on the fabric, the high molecular weight compounds are added to the wash liquor, e.g.. carboxymethyl cellulose.
The mechanism of effective removal of dirt and preventing the repeated deposition of dirt and other agents and by-products, formed in the washing bath on the surface of the washed material, in currently used laundry detergents, it is very complex. The number of ingredients that make up the wash bath is also significant.